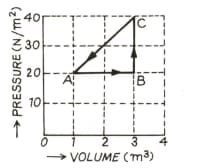
In the adjoining diagram are shown the changes taking place in a thermodynamic system in going from the initial state to the states and and finally returning to the state If joule, joule and the heat spent in the change from state to is joule, then determine the value of .


Important Questions on Internal Energy : First Law of Thermodynamics : Specific Heat
The initial pressure and volume of a gas are respectively. They are increased to In which process will more work have to be done : (1) first increasing the volume only and increasing the pressure, or (2) first increasing the pressure only and then increasing the volume? Will the change in internal energy of the gas in these process be different?
What do you understand by cyclic process?
What do you mean by internal energy of a gas?
Why is internal energy said to be a unique function?
Prove that the difference in the molar specific heats of an ideal gas is nearly Given :
What is meant by 'degrees of freedom' of a gas molecule?
Steam at is passed into of water at when water acquires a temperature of , the mass of water present will be :
[Take specific heat of water and latent heat of steam]
When a system is taken from state to state along the path , it is found that Along the path . along the path

