MEDIUM
JEE Main
IMPORTANT
Earn 100
In which of the following solutions, permits the solvent molecules to pass through?
(a)Benzoic acid in benzene.
(b)Urea in acetone.
(c)Urea in water.
(d)Thiourea in water.
50% studentsanswered this correctly

Important Questions on Solutions
EASY
JEE Main
IMPORTANT
Which statement(s) is/are correct about osmotic pressure (), volume () and temperature ()?
HARD
JEE Main
IMPORTANT
The slope of vs was made against insulin concentration in and temperature . If is in and the slope of line obtained is , which of the following are not the molar mass (in ) of insulin?
MEDIUM
JEE Main
IMPORTANT
A graph showing the variation of osmotic pressure versus molar concentration of an aqueous solution at temperature is given below.
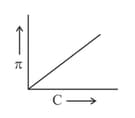
The slope of the line doesn't represent
MEDIUM
JEE Main
IMPORTANT
Calculate the osmotic pressure of (mass/volume anhydrous) solution at assuming ionisation in .
EASY
JEE Main
IMPORTANT
Under what conditions Van't Hoff factor is equal to unity?
EASY
JEE Main
IMPORTANT
Under what conditions van't Hoff factor is less than ?
EASY
JEE Main
IMPORTANT
Under what conditions van't Hoff factor is greater than ? Explain your answer.
MEDIUM
JEE Main
IMPORTANT
If the solution taken is too concentrated, then how will it affect the molar mass of the solute?
