Ions like and are present in a solution of sodium chloride.
Which are the ions attracted to the positive electrode?

Important Questions on Reactivity Series and Electrochemistry
Which are the ions attracted to the negative electrode?
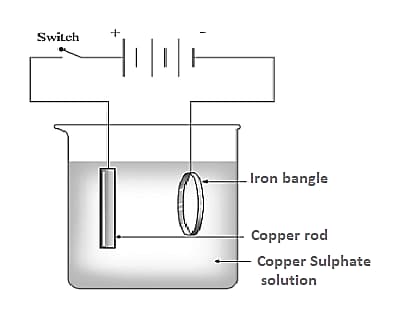
Observe the picture given. This is the process of electroplating of copper on iron bangle.
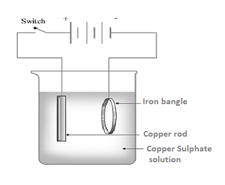
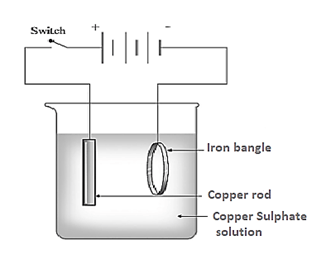
Which metal is connected to the negative terminal of the battery?
Observe the picture given. This is the process of electroplating of copper on an iron bangle.
Which metal is connected to the positive terminal of the battery?
Observe the picture given. This is the process of electroplating of copper on iron bangle.
Which solution is used as electrolyte?
The solution of and are taken on three different test tube. Suppose, an iron nail is kept immersed in each one.
- In which test tube the iron nail undergoes a colour change?
- What is the reaction taking place?
- Justify your answer.
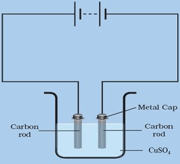
Keep two carbon rods immersed in a copper sulphate solution. Then pass the electricity through the same solution.
At which electrode does colour change occur- (anode or cathode)