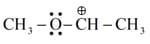MEDIUM
11th CBSE
IMPORTANT
Earn 100
Match Column I with Column II.
Column I
Column II
(i)
Dumas method
(a)
(ii)
Kjeldahl's method
(b)
Silica gel
(iii)
Carius method
(c)
Nitrogen gas
(iv)
Chromatography
(d)
Free radicals
(v)
Homolysis
(e)
Ammonium sulphate

Important Questions on Organic Chemistry – Some Basic Principles and Techniques
MEDIUM
11th CBSE
IMPORTANT
Match the intermediates given in Column I with their probable structure in Column II.
| Column I | Column II | ||
| (i) | Free radical | (a) | Trigonal planar |
| (ii) | Carbocation | (b) | Pyramidal |
| (iii) | Carbanion | (c) | Linear |
HARD
11th CBSE
IMPORTANT
Match the ions given in Column I with their nature-given in Column II.
| Column I | Column II | ||
| (i) |
|
(a) | Stable due to resonance |
| (ii) | (b) | Destabilised due to inductive effect | |
| (iii) | 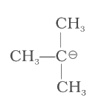 |
(c) | Stabilised by hyperconjugation |
| (iv) | (d) | A secondary carbocation |
HARD
11th CBSE
IMPORTANT
What is meant by hybridisation? Compound contains or hybridised carbon atoms. Will it be a planar molecule?
HARD
11th CBSE
IMPORTANT
Benzoic acid is an organic compound. Its crude sample can be purified by crystallisation from hot water. What characteristic differences in the properties of benzoic acid and the impurity make this process of purification suitable?
HARD
11th CBSE
IMPORTANT
Two liquids can be separated by the method of fractional distillation. The boiling point of a liquid is less than the boiling point of a liquid . Which of the liquids do you expect to come out first in the distillate? Explain.
HARD
11th CBSE
IMPORTANT
You have a mixture of three liquids . There is a large difference in the boiling points of and rest of the two liquids i.e., . The boiling point of liquids are quite close. Liquid boils at a higher temperature than and boiling point of is lower than . How will you separate the components of the mixture? Draw a diagram showing set up of the apparatus for the process.
HARD
11th CBSE
IMPORTANT
Draw a diagram of bubble plate type fractionating column. When do we require such type of column for separating two liquids? Explain the principle involved in the separation of components of a mixture of liquids by using a fractionating column. What industrial applications does this process have?
HARD
11th CBSE
IMPORTANT
A liquid with high boiling point decomposes on simple distillation but it can be steam distilled for its purification. Explain how is it possible?