EASY
MHT-CET
IMPORTANT
Earn 100
Metallic lustre is explained by
(a)diffusion of metal ions.
(b)oscillation of loose electrons.
(c)Excitation of free protons.
(d)existence of lattice.
52.17% studentsanswered this correctly

Important Questions on Solid State
EASY
MHT-CET
IMPORTANT
EASY
MHT-CET
IMPORTANT
EASY
MHT-CET
IMPORTANT
EASY
MHT-CET
IMPORTANT
What is the total volume of atoms present in a face-centre cubic unit cell of a metal ( is the atomic radius)?
If the answer is of type , report the value of in the simplest fraction.
EASY
MHT-CET
IMPORTANT
EASY
MHT-CET
IMPORTANT
EASY
MHT-CET
IMPORTANT
EASY
MHT-CET
IMPORTANT
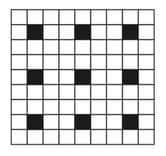
A two-dimensional solid pattern formed by two different atoms and is shown below. The black and white squares represent atoms and , respectively. The simplest formula for the compound based on the unit cell from the pattern is