HARD
JEE Advanced
IMPORTANT
Earn 100
One mole of a monoatomic real gas satisfied the equation where b is a constant. The relationship of interatomic potential V(r) and interatomic distance r for the gas is given by
(a)
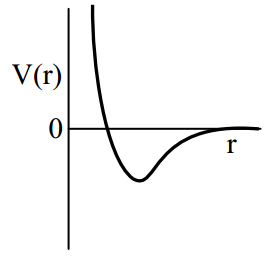

(b)
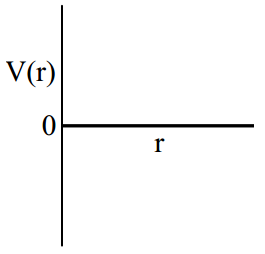
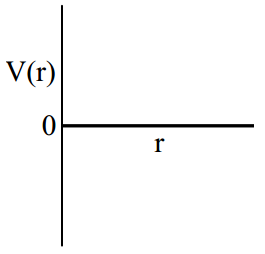
(c)
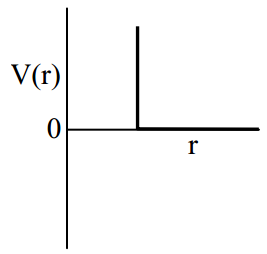
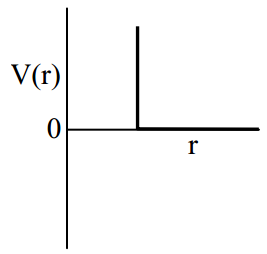
(d)
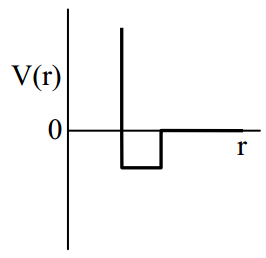
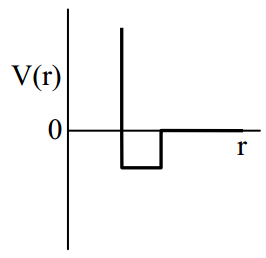
50% studentsanswered this correctly

Important Questions on States of Matter: Gases and Liquids
HARD
JEE Advanced
IMPORTANT
In thermodynamics, the work done is given by . For a system undergoing a particular process, the work done is,
. This equation is applicable to a
HARD
JEE Advanced
IMPORTANT
Aluminium reacts with sulfuric acid to form aluminium sulfate and hydrogen. What is the volume of hydrogen gas in liters produced at and atm pressure, when of aluminium and of sulfuric acid are combined for the reaction? (Use molar mass of aluminium as , )
HARD
JEE Advanced
IMPORTANT
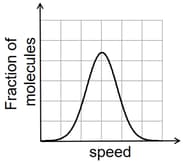
If the distribution of molecular speeds of a gas is as per the figure shown below, then the ratio of the most probable, the average, and the root mean square speeds, respectively, is
MEDIUM
JEE Advanced
IMPORTANT
Which of the following statement(s) (are) correct regarding the root-mean-square speed and average translational kinetic energy of a molecule in a gas at equilibrium?
