MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
One mole of a real gas is subjected to heating at constant volume from state to state. Then it is subjected to irrerversible adiabatic compression against constant external pressure till system reaches final state . If the constant volume molar heat capacity of real gas is . Find out correct expression for from state to state .
(a)
(b)
(c)
(d)
66.67% studentsanswered this correctly

Important Questions on Thermodynamics
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
What can be concluded about the value of and from this graph?
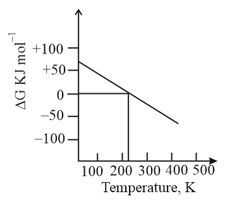
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
