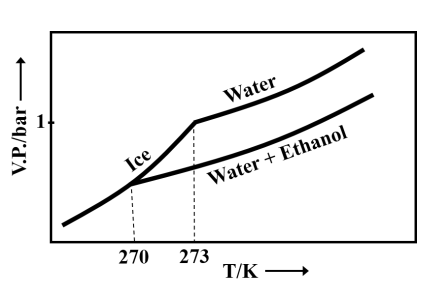
Pure water freezes at and pressure. The addition of of ethanol to of water changes the freezing point of the solution. Use the freezing point depression constant of water as . The figures shown below represent the plots of vapour pressure () versus temperature (). [Molecular weight of ethanol is 46 g ]
Among the following, the option representing a change in the freezing point is
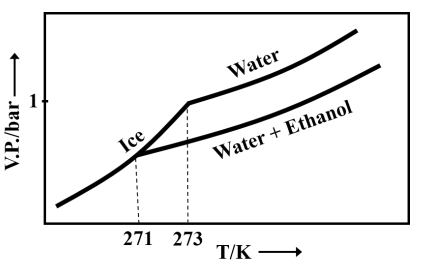
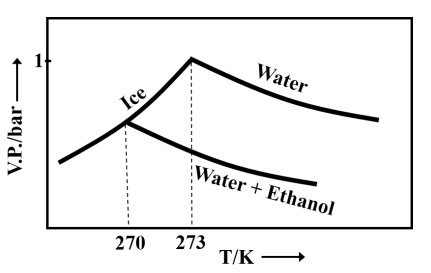
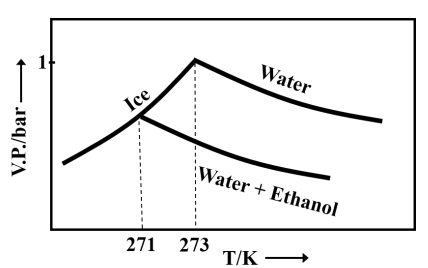

Important Questions on Solutions
On dissolving of a non-volatile non-ionic solute to of benzene, its vapor pressure decreases from to The depression of freezing point of benzene (in ) upon addition of the solute is _______
(Given data: Molar mass and the molal freezing point depression constant of benzene are and respectively)
Give your answer up to three significant figures.
(Given that the vapour pressure of pure liquid A is at temperature )
The plot given below shows curves (where, is the pressure and is the temperature) for two solvents and and isomolal solutions of in these solvents. completely dissociates in both the solvents.
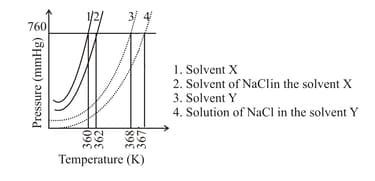
On the addition of equal number of moles of a non-volatile solute in equal amount (in ) of these solvents, the elevation of boiling point of solvent is three times that of solvent . The solute is known to undergo dimerization in these solvents. If the degree of dimerization is in the solvent , the degree of dimerization in the solvent is _______.