HARD
JEE Main
IMPORTANT
Earn 100
Saponification of ethyl acetate by is studied by titration of the reaction mixture having molar ratio of the reactants. If of is required by of the solution at the start and of is required by another after minutes, then the rate constant is-
(a)
(b)
(c)
(d)
26.42% studentsanswered this correctly

Important Questions on Chemical Kinetics
HARD
JEE Main
IMPORTANT
Consider the decay of to and by two parallel first-order reactions as shown in the figure
| Reaction | Rate constant | Energy of activation | |
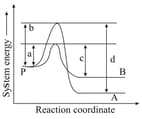
Which of the following is (are) true?
MEDIUM
JEE Main
IMPORTANT
Which of the following statements are correct?
HARD
JEE Main
IMPORTANT
In the reversible reaction, the rate of disappearance of is equal to-
HARD
JEE Main
IMPORTANT
The rate expression for the reaction,
can be derived from the mechanism,
Which of the following statements are correct about rate expression?
HARD
JEE Main
IMPORTANT
The rate of formation of for the forward reaction, is first-order with respect to and each. Which among the following are correct?
