MEDIUM
JEE Main
IMPORTANT
Earn 100
Select the incorrect statements about Ellingham diagram.
(a)Theoretically, all oxides cannot be decomposed to give the metal and dioxygen if a sufficiently high temperature can be attained.
(b)Any metal will not reduce the oxide of other metals which lie above it in the Ellingham diagram.
(c)When temperature is raised, a point will be reached where the graph crosses the line. Below this temperature, the free energy of formation of the oxide is negative, so the oxide is stable.
(d)According to Ellingham diagram, Al will not reduce at temperature below .
50% studentsanswered this correctly

Important Questions on General Principles and Processes of Isolation of Elements
HARD
JEE Main
IMPORTANT
Why an external more than is required for the extraction of from brine?
EASY
JEE Main
IMPORTANT
Write the two basic requirements for refining of metal by Mond's process and by van Arkel method.
EASY
JEE Main
IMPORTANT
A mixture of compounds and is passed through a column of aluminium oxide by using alcohol as eluent. Compound is eluted in preference to compound . Which of the compounds ( or ) is more readily adsorbed on the column?
EASY
JEE Main
IMPORTANT
How are metals that are used as semiconductors refined? What is the principle of the method used for refining metals like germanium, silicon, etc.?
EASY
JEE Main
IMPORTANT
When an inert atmosphere is required for a metallurgical process, nitrogen is generally used. However, during the reduction of by magnesium, helium is preferred. Explain.
EASY
JEE Main
IMPORTANT
Why metal ores are not found in their nitrate form?
MEDIUM
JEE Main
IMPORTANT
Consider the following chromatogram (column chromatography).
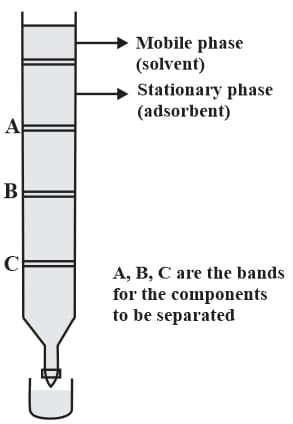
Which substance can act as stationary phase?
MEDIUM
JEE Main
IMPORTANT
Consider the following chromatogram (column chromatography).
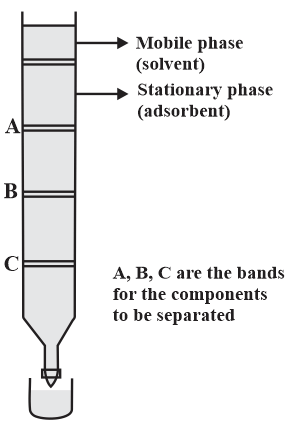
Which of the three components ( or ) is eluted first?
