Sodium metal crystallizes in a body-centred cubic lattice with the cell . The radius (in ) of sodium atom is

Important Questions on Solid State
Which of the following shaded plane in FCC lattice contains arrangement of atoms as shown below?
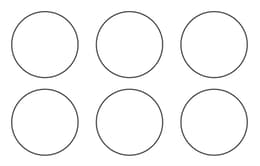
Given is the arrangement of atoms in a crystallographic plane. Which plane correctly represent(s) the drawn structure?
In crystalline solids, atoms or molecules are arranged in regular and long range order fashion in three dimensional pattern. These have sharp melting point, flat faces, sharp edges, bounded by well defined planes. A large number of unit cells, each of which possess a definite geometry bounded by plane faces give rise to the formation of crystal. A point at the corner of unit cell contributes for of each such point of unit cell. A point along the edge contributes for of each such point to unit cell. A body centred point contributes for 1 of such point of unit cell. Coordination number is the number of neighbours that each ion is surrounded by an oppositely charged ions. Radius of the unit cell in sc, fcc and bcc is where is the edge length of the unit cell.
An alloy of and gold crystallizes in cubic lattice in which the gold atoms occupy the lattice points at the corners of cube and copper atoms occupy the center of each face. The formula of this compound is
