Sodium metal is not refined by the electrolytic reduction of aqueous solution of sodium sulphate but copper is refined by electrolytic reduction of copper sulphate. Explain these statements in the light of the activity series of metals.

Important Questions on Metals and Nonmetals
Write a balanced chemical equation to describe the reaction between sodium metal and cold water. What would be observed when
(i) a burning candle is brought near the reaction mixture.
(ii) four drops of phenolphthalein are added in the reaction mixture.
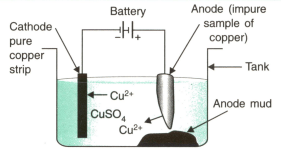
The given figure is the experimental setup to refine copper metal. Answer the following questions:
(i) Which material is used as anode?
(ii) In which direction do ions move in the electrolytic cell?
(iii) Name three impurities which collect as anode mud.
Nonmetal A, a major gaseous component of air, combines with in the mole ratio in the presence of iron catalyst to give a gas B which is highly soluble in water. Gas A forms a reddish brown oxide C, When C is dissolved in water in the presence of air an acid D is formed which is a strong oxidizing agent.
(i) Identify A, B, C and D
(ii) What is the nature of the aqueous solution of gas B?
(iii) To which group and period of the period table does the nonmetal A belong?
Roasting of .
