EASY
10th CBSE
IMPORTANT
Earn 100
Solid calcium oxide was taken in a container and water was added slowly to it.
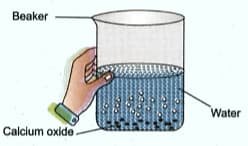
Write the name and the chemical formula of the product formed.

Write the name and the chemical formula of the product formed.

Important Questions on Chemical Reactions and Equations
MEDIUM
10th CBSE
IMPORTANT
(a) What is the colour of copper sulphate crystals
(i) before heating and
(ii) After heating.
EASY
10th CBSE
IMPORTANT
What is the source of liquid droplets seen on the inner upper side of the test tube during the heating process?
MEDIUM
10th CBSE
IMPORTANT
Write the balanced chemical equation for the following reaction:
Zinc carbonate (s) Zinc oxide (s) + Carbon dioxide (g).
MEDIUM
10th CBSE
IMPORTANT
What are the displacement reactions? Give one example.
MEDIUM
10th CBSE
IMPORTANT
What are exothermic reactions? Give one example.
MEDIUM
10th CBSE
IMPORTANT
Write chemical equation for the following reaction: When solid mercury(II) oxide is heated, liquid mercury and oxygen gas are produced.
MEDIUM
10th CBSE
IMPORTANT
Select the oxidising agent and reducing agent in the reaction:
MEDIUM
10th CBSE
IMPORTANT
When sulphur dioxide is passed through a saturated solution of hydrogen sulphide, the following reaction occurs:
Predict the reducing agent and oxidising agent in this reaction.
