MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
Some energy levels of a molecule are shown in the figure. The ratio of the wavelengths is given by :
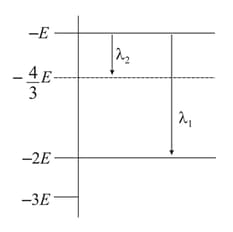

(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Atomic Physics
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
Suppose in certain conditions only those transitions are allowed to hydrogen atoms in which the principal quantum number change by .
(i) Find the smallest wavelength emitted by hydrogen
(ii) List the wavelengths emitted by hydrogen in the visible range ( to ) ()
HARD
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
