MEDIUM
Earn 100
Statement - : Only temperature can change the vapour pressure of a pure liquid.
Statement - : Equilibrium constant does not change unless temperature is changed.
(a)Statement-, is True, Statement - is True; Statement - is a correct explanation for Statement-.
(b)Statement - is True, Statement is True; Statement - , is NOT a correct explanation for Statement - .
(c)Statement - is True, Statement - , is False.
(d)Statement - is False, Statement - is True.
42.86% studentsanswered this correctly
Important Questions on Solutions
MEDIUM
HARD
EASY
MEDIUM
(
)
MEDIUM
Which of the following relations is correct?
MEDIUM
HARD
(Given that the vapour pressure of pure liquid A is at temperature )
MEDIUM
MEDIUM
EASY
MEDIUM
(molar mass of urea )
EASY
EASY
HARD
EASY
EASY
MEDIUM
MEDIUM
The vapour pressure vs. temperature curve for a solution solvent system is shown below.
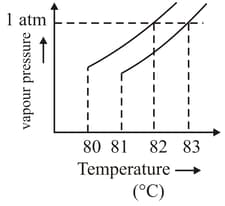
The boiling point of the solvent is _____°C.
EASY
MEDIUM

