Substance reacts with dilute violently, reacts slowly while does not react at all. and could be respectively

Important Questions on Metals and Non-Metals
(i)
(ii)
(iii)
(iv)
Match column I with column II and select the correct option from the given codes.
| Column I | Column II | ||
| (a) | A non-metal, essential for respiration | (i) | Graphite |
| (b) | A non-metal, the source of energy in Sun | (ii) | Silicon |
| (c) | A non-metal, good conductor of electricity | (iii) | Oxygen |
| (d) | A non-metal, used in semiconductors | (iv) | Hydrogen |
Vidisha placed a copper wire in silver nitrate solution as shown in the figure.
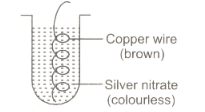
Which of the following represents the correct observation?
Read the following statements carefully and identify and respectively.
(i) is stored under kerosene.
(ii) catches fire on exposure to air and stored in water.
(iii) reacts with steam.
Read the given statements and select the correct option.
Statement 1: Silver objects become green and lose their shine with the passage of time.
Statement 2: Silver reacts with carbon dioxide and moisture present in the atmosphere.
