MEDIUM
JEE Main
IMPORTANT
Earn 100
Suppose that a hypothetical atom gives a red, green, blue and violet line in the spectrum. Which jump according to figure would give off the red spectral line?
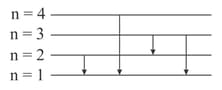

(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Atomic Structure
MEDIUM
JEE Main
IMPORTANT
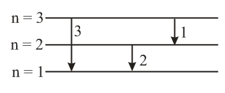
For the following transitions in hydrogen-like atoms, select the correct relation(s):
HARD
JEE Main
IMPORTANT
If the electron of the hydrogen atom is replaced by another particle of the same charge but of the double mass, then:
HARD
JEE Main
IMPORTANT
Let and are the radius of the orbit, speed of the electron and the total energy of the electron, respectively. Which of the following quantities are proportional to the quantum number
MEDIUM
JEE Main
IMPORTANT
Select the correct statements for hydrogen like atoms or ions.
HARD
JEE Main
IMPORTANT
An electron in a hydrogen atom makes a transition from to The time period of the electron in the initial state is eight times that in the final state. What are the possible values of and
MEDIUM
JEE Main
IMPORTANT
Identify the correct statement(s) from the following:
HARD
JEE Main
IMPORTANT
A hydrogen-like atom has ground state binding energy . Then:
MEDIUM
JEE Main
IMPORTANT
What is the ratio of the velocities of and molecules so that they are associated with de-Broglie waves of equal wavelength?
