EASY
NEET
IMPORTANT
Earn 100
The attractive potential energy between electron and nucleus is given by and are constants and is the radius. The radius of the nth Bohr's orbit depends upon principal quantum number as:
7.69% studentsanswered this correctly
Important Questions on Atomic Physics
MEDIUM
NEET
IMPORTANT
HARD
NEET
IMPORTANT
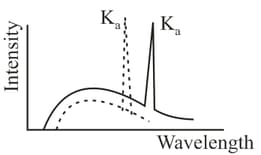
Figure shows the intensity-wavelength relations of -rays coming from two different Coolidge tubes. The solid curve represents the relation for the tube in which the potential difference between the target and the filament is and the atomic number of the target material is These quantities are and for the other tube. Then,
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
Assertion: Neutrons penetrate matter more readily as compared to protons.
Reason: Neutrons are slightly more massive than protons.
