EASY
Agniveer Vayu
IMPORTANT
Earn 100
The concept that originates from Zeroth law of thermodynamics is
(a)internal energy
(b)heat content
(c)pressure
(d)temperature
57.14% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
Agniveer Vayu
IMPORTANT
When an ideal diatomic gas is heated at constant pressure, the fraction of the heat energy supplied which increases the internal energy of the gas is
EASY
Agniveer Vayu
IMPORTANT
Assertion: It is possible that there is a change in temperature of a body without giving or taking heat from the system.
Reason: Internal energy is a function of temperature.
EASY
Agniveer Vayu
IMPORTANT
Assertion: In the process , if the volume of gas increases, then work done by gas decreases.
Reason: Work done in the above process is
EASY
Agniveer Vayu
IMPORTANT
monoatomic gas of density and pressure is enclosed in a container. Internal energy of gas will be:
EASY
Agniveer Vayu
IMPORTANT
of a monoatomic gas is at a pressure of . The density of the gas is . What is the order of energy of the gas due to its thermal motion?
EASY
Agniveer Vayu
IMPORTANT
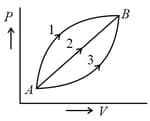
In the figure, a certain mass of gas traces three paths from state to state . If work done by the gas along three paths are respectively, then
EASY
Agniveer Vayu
IMPORTANT
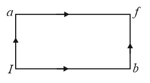
When a system is taken from state to state along the path , it is found that and . Along the path undefined . along the path is
EASY
Agniveer Vayu
IMPORTANT
An ideal gas is taken around the cycle as shown in the diagram
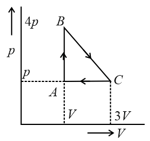
The total work done by the gas during the cycle is
