EASY
JEE Main
IMPORTANT
Earn 100
The crystals are bounded by plane faces , straight edges , and interfacial angle . The relationship between these is
50% studentsanswered this correctly
Important Questions on Solid State
EASY
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
For the structure given below, the site marked as is a:
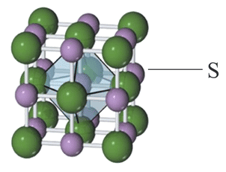
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
EASY
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
