EASY
JEE Main
IMPORTANT
Earn 100
The curve of binding energy per nucleon as a function of atomic mass number has a sharp peak for helium nucleus. This implies that helium,
(a)Can easily be broken up
(b)Is very stable
(c)Can be used as fissionable material
(d)Is radioactive
100% studentsanswered this correctly

Important Questions on Nuclear Physics
HARD
JEE Main
IMPORTANT
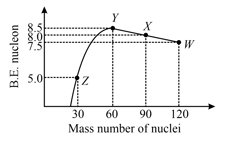
Binding energy per nucleon verses mass number curve for nuclei is shown in the figure. , , and are four nuclei indicated on the curve. The process that would release the energy is,
EASY
JEE Main
IMPORTANT
The binding energy per nucleon is maximum in the case of,
MEDIUM
JEE Main
IMPORTANT
The masses of neutrons and protons are and , respectively. If the neutrons and protons combine to form a Helium nucleus (-particles) of mass , then, the binding energy of the Helium nucleus will be (),
MEDIUM
JEE Main
IMPORTANT
The mass defect for the nucleus of helium is . What is the binding energy per nucleon for helium in ?
EASY
JEE Main
IMPORTANT
denotes the mass of a proton and that of a neutron. A given nucleus, of binding energy , contains protons and neutrons. The mass of the nucleus is given by ( is the velocity of light)
EASY
JEE Main
IMPORTANT
The binding energy of deuteron is per nucleon and an -particle has a binding energy of per nucleon. Then in the fusion reaction the energy released is,
EASY
JEE Main
IMPORTANT
If the binding energy per nucleon in and nuclei are, respectively, and then energy of reaction is,
EASY
JEE Main
IMPORTANT
The binding energy per nucleon of deuterium and helium atom is and . If two deuterium nuclei fuse to form helium atom, the energy released is,
