EASY
JEE Main
IMPORTANT
Earn 100
The degree of dissociation of an electrolyte does not depend on
(a)the nature of the electrolyte.
(b)the catalyst.
(c)the dilution.
(d)the temperature.
50% studentsanswered this correctly

Important Questions on Miscellaneous Objective Questions
MEDIUM
JEE Main
IMPORTANT
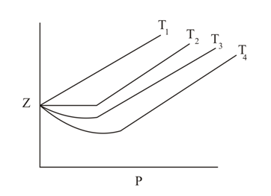
From the given plot between and for a real gas, the correct relation is:
MEDIUM
JEE Main
IMPORTANT
The van der Waals equation for mole of gas is, where,
MEDIUM
JEE Main
IMPORTANT
A mixture of and taken in a bulb in mole ratio is allowed to diffuse through a fine hole. The composition of the gases (mole ratio) coming out initially will be:
MEDIUM
JEE Main
IMPORTANT
For a dilute solution, or, , where is:
EASY
JEE Main
IMPORTANT
For ideal binary solution,
This equation reflects
HARD
JEE Main
IMPORTANT
Dry air is passed through a solution and then through its solvent and finally through . The ratio of weight loss in the solvent to the weight gain in gives
MEDIUM
JEE Main
IMPORTANT
The relative lowering of vapour pressure of an aqueous solution of urea is . If for is the elevation in boiling point will be:
MEDIUM
JEE Main
IMPORTANT
mole of is allowed to decompose in a litre container. mole of was produced at equilibrium. The equilibrium constant for the equilibrium is
