MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
The density in grams per litre of ethylene at STP is
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Kinetic Theory of Gases
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
Pressure versus temperature graph of an ideal gas is shown in figure. Density of the gas at point is . Density at will be:-
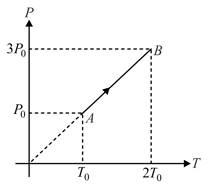
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
A vessel is partitioned in two equal halves by a fixed diathermic separator. Two different ideal gases are filled in left and right halves. The root-mean-square speed of the molecules in part is equal to the mean speed of molecules in the part. Then the ratio of the mass of a molecule in part to that of a molecule in part is:-
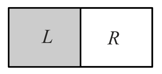
HARD
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
