MEDIUM
JEE Main
IMPORTANT
Earn 100
The density of (fluorite structure) is . The length of the side of the unit cell is
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Solid State
HARD
JEE Main
IMPORTANT
HARD
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
EASY
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
HARD
JEE Main
IMPORTANT
Copper has a face-centred cubic structure with a unit-cell edge length of . What is the size of the largest atom (in pm), which could fit into the interstices of the copper lattice without distorting it?
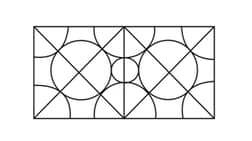
[Hint: Calculate the radius of the smallest circle in the figure.]
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
