The diagram shows the pressure and volume relationship for one cycle of operation of an engine.
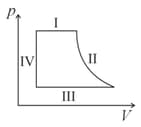
Which of the labelled parts of the cycle identify isobaric changes and adiabatic changes of state?

Important Questions on Chemical Thermodynamics
Reversible process
The total number of intensive properties from the following is _____.
Volume, Molar heat capacity, molarity, ,Gibbs free energy change, Molar mass, Mole
Among the following the number of state variable is
Internal energy
Volume
Heat
Enthalpy
Observe the following properties:
Volume, enthalpy, density, temperature, heat capacity, pressure, internal energy.
The number of extensive properties in the above list is
One mole of an ideal monoatomic gas undergoes two reversible processes and as shown in the given figure:
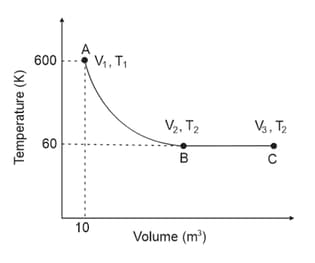
is an adiabatic process. If the total heat absorbed in the entire process and is , the value of is
[Use molar heat capacity of the gas at constant pressure, ]
(: pressure, : volume, : temperature, : enthalpy, : entropy)
i)
ii)
iii)
iv)
