MEDIUM
JEE Main
IMPORTANT
Earn 100
The equivalent volume of in the following reaction is:
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Miscellaneous Objective Questions
EASY
JEE Main
IMPORTANT
The equivalent volume of in the following reaction is:
MEDIUM
JEE Main
IMPORTANT
One coulomb is the charge of:
EASY
JEE Main
IMPORTANT
For a zero-order reaction, with the initial reactant concentration , the time for completion of the reaction is:
MEDIUM
JEE Main
IMPORTANT
Which statement (s) about the behaviour of a real gas is (are) wrong?
MEDIUM
JEE Main
IMPORTANT
The figure shows the effect of pressure on the compressibility factor, , of a gas.
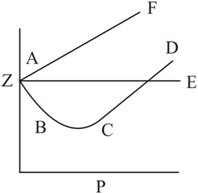
The wrong conclusion (s) is (are):
MEDIUM
JEE Main
IMPORTANT
Equivalent weights of in the following reactions:
and
, are respectively:
EASY
JEE Main
IMPORTANT
According to the Faraday's laws of electrolysis, the discharge of one electrochemical equivalent of ions should involve:
EASY
JEE Main
IMPORTANT
In which of the following cases a gas is more compressible than the ideal gas?
