MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
The following graph shows two isotherms for a fixed mass of an ideal gas. Find the ratio of rms speed of the molecules at temperatures and ?
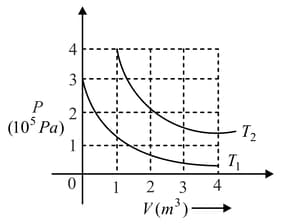


Important Questions on Kinetic Theory of Gases
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
The figure shows graphs of pressure vs density for an ideal gas at two temperatures and .
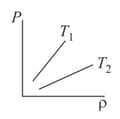
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
Four containers are filled with monoatomic ideal gases. For each container, the number of moles, the mass of an individual atom and the r.m.s. speed of the atoms are expressed in terms of and , respectively. If and are their temperatures, respectively, then which one of the options correctly represent the order?
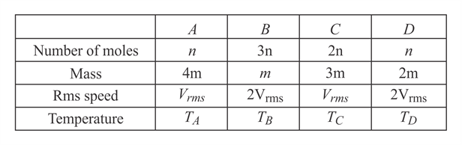
HARD
JEE Main/Advance
IMPORTANT
