MEDIUM
NEET
IMPORTANT
Earn 100
The instantaneous rate of disappearance of ion in the following reaction is .
What is the rate of appearance of ?
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Chemical Kinetics
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
For a first-order reaction, the concentration of and at the point of intersection is given in the figure:
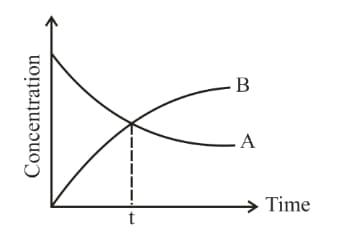
If mol at the time is equal to:
