EASY
JEE Main/Advance
IMPORTANT
Earn 100
The internal energy of a gas in an adiabatic process is given by , find
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
JEE Main/Advance
IMPORTANT
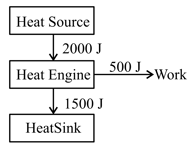
What would be the efficiency of the heat engine diagramed as shown below?
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
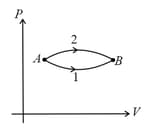
The figure shows two paths for the change of state of a gas from to . The ratio of molar heat capacities in path and path is:-
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
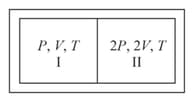
A partition divides a container having insulated walls into two compartments and . The same gas fills the two compartments whose initial parameters are given. The partition is a conducting wall which can move freely without friction. Which of the following statements is/are correct, with reference to the final equilibrium position?
HARD
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
