EASY
NEET
IMPORTANT
Earn 100
The internal energy of a mono-atomic gas is,
50% studentsanswered this correctly
Important Questions on Kinetic Theory of Gases
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
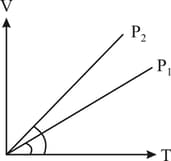
In the following diagram what is the relation between and
EASY
NEET
IMPORTANT
A certain mass of an ideal gas undergoes a reversible isothermal compression. When compared with their initial state, its molecules will have the same
(i) root mean square velocity
(ii) mean momentum
(iii) mean kinetic energy
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
