The net enthalpy change of a reaction is the amount of energy required to break all the bonds in reactant molecules minus amount of energy required to form all the bonds in the product molecules. What will be the enthalpy change for the following reaction.
Given that Bond energy of and is and respectively.

Important Questions on Thermodynamics
The enthalpy of reaction for the reaction:
is . What will be standard enthalpy of formation of ?
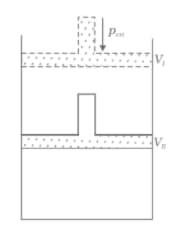
What will be the work done on an ideal gas enclosed in a cylinder, when it is compressed by a constant external pressure, in a single step as shown in Figure? Explain graphically.
Represent the potential energy/enthalpy change in the following processes graphically.
(a) Throwing a stone from the ground to roof.
(b)
In which of the processes potential energy/enthalpy change is contributing factor to the spontaneity?
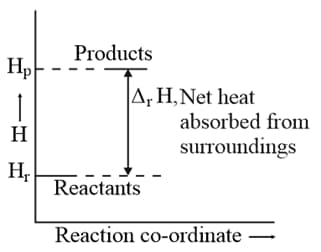
Enthalpy diagram for a particular reaction is given in Figure. Is it possible to decide spontaneity of a reaction from given diagram? Explain.
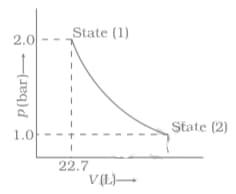
mol of a monoatomic ideal gas is expanded from state () to state () as shown in Figure. Calculate the work done for the expansion of gas from state () to state () at .