EASY
JEE Main
IMPORTANT
Earn 100
The normality of phosphorous acid is
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Solutions
EASY
JEE Main
IMPORTANT
What mass of ethanol be added to of water to have the mole fraction of ethanol equal to ?
EASY
JEE Main
IMPORTANT
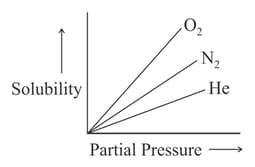
Molar solubility of helium, nitrogen and oxygen are plotted against partial pressure of the gas at constant temperature. Henry's law constant for these gases will lie in which of the following sequences?
MEDIUM
JEE Main
IMPORTANT
According to William Henry, the solubility of a gas in liquid depends on the pressure of the gas. If '' is the molality of the gas and '' is its pressure, then which of the following plot is in accordance with the law?
MEDIUM
JEE Main
IMPORTANT
Which of the following plots is correct for solutions of different solutes having the same density?
is molarity and is molar mass of the solute.
EASY
JEE Main
IMPORTANT
The molarity of a solution obtained by mixing of with of will be:
MEDIUM
JEE Main
IMPORTANT
The vapour pressure of a dilute solution of a solute is influenced by
MEDIUM
JEE Main
IMPORTANT
By adding water to the solution of an ionic compound, its
HARD
JEE Main
IMPORTANT
For a binary ideal liquid solution, the variation in total vapuor pressure versus composition of solution is given by which of the curves?
