MEDIUM
JEE Main
IMPORTANT
Earn 100
The for acetylsalicylic acid (aspirin) is The of gastric juice in the human stomach is about and the in the small intestine is about . Aspirin will be:
(a)un-ionised in the small intestine and in the stomach.
(b)completely ionised in the small intestine and in the stomach.
(c)ionised in the stomach and almost un-ionised in the small intestine.
(d)ionised in the small intestine and almost un-ionised in the stomach.
50% studentsanswered this correctly

Important Questions on Miscellaneous Objective Questions
EASY
JEE Main
IMPORTANT
The degree of dissociation of an electrolyte does not depend on
MEDIUM
JEE Main
IMPORTANT
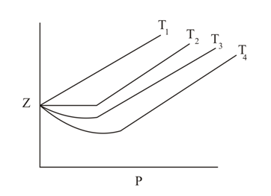
From the given plot between and for a real gas, the correct relation is:
MEDIUM
JEE Main
IMPORTANT
The van der Waals equation for mole of gas is, where,
MEDIUM
JEE Main
IMPORTANT
A mixture of and taken in a bulb in mole ratio is allowed to diffuse through a fine hole. The composition of the gases (mole ratio) coming out initially will be:
MEDIUM
JEE Main
IMPORTANT
For a dilute solution, or, , where is:
EASY
JEE Main
IMPORTANT
For ideal binary solution,
This equation reflects
HARD
JEE Main
IMPORTANT
Dry air is passed through a solution and then through its solvent and finally through . The ratio of weight loss in the solvent to the weight gain in gives
MEDIUM
JEE Main
IMPORTANT
The relative lowering of vapour pressure of an aqueous solution of urea is . If for is the elevation in boiling point will be:
