The percentage ionic character of a single bond may be estimated from ratio of the observed dipole moment to the calculated moment, assuming oppositely charged ions located at a distance equal to the bond length. The observed dipole moments of and are and , respectively. Calculate the percentage ionic character of the bond in each of these compounds. Do the results obtained are parallel to the magnitudes of the extra ionic resonance energies, , for these molecules?
(Given, then ).

Important Questions on Chemical Bonding and Molecular Structure
Arrange the following compounds in decreasing order of dipole moment values. Explain the order.
Arrange the following compounds in decreasing order of dipole moment values. Explain the order.
Arrange the following compounds in decreasing order of dipole moment values. Explain the order.
Arrange the following compounds in decreasing order of dipole moment values. Explain the order.
Assign orientation of the three chloronitrobenzene(ortho, meta, para) with and .
Ortho-isomers have dipole moments. In which cases dipole moments are maximum and minimum?
The experimental value of the dipole moment of is . The length of the bond is . The percentage ionic character in is:
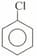 is . The dipole moment of
is . The dipole moment of 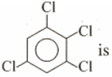 .
.