MEDIUM
NEET
IMPORTANT
Earn 100
The ratio between the velocity of at and that of at is:
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on States of Matter: Gases and Liquids
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
An ideal gas is subjected to a cyclic change as shown in the diagram below:
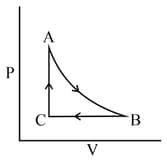
The step in which the gas will cool down is along:
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
The volume vs. temperature graph of mole of an ideal gas is given below. The pressure of the gas (in ) at and , respectively, are

