The reaction (aq) is monitored by measuring optical rotation of reaction mixture at different time interval. The species $A, B$ and $C$ are optically active with specific rotations and Respectively. Starting with pure A if the value of optical rotations was found to be after Minutes and optical rotations was After infinite time. Find the rate constant for order conversion of A into B and C.

Important Questions on Chemical Kinetics
Isomerization of cyclobutene into 1,3 -butadine follow first order kinetics as :
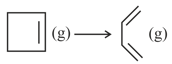
The kinetic study was performed by taking same amounts of cyclobutene in three sealed flasks. First flask was broken after 20 minute and the reaction mixture was absorbed completely in bromine solution. bromine solution was required. The second flask was broken after a very long time and the reaction mixture required bromine solution of the same strength. If the third flask was broken after 30 minute, what volume of bromine solution of same strength would have been required?
Decomposition of both and Follows 1st order kinetic as :
If one mole of each and Are taken in a Evacuated flask and heated to some temperature so that they start decomposing at the same rate, determine total pressure in the flask after .
At room temperature orange juice gets spoilt in about 64 hours. In a refrigerator at juice can be stored three times as long before it gets spoilt. Estimate
(a) the activation energy of the reaction that causes the spoiling of juice.
A certain organic compound A decomposes by two parallel first order mechanism
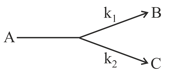
If and
Calculate the concentration ratio of to , if experiment is started with only and allowed to run for one hour.
