The reaction proceeds in parallel channels
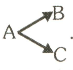
Suppose the half life values for the two branches are minutes and minutes, what is the overall half-life value?


Important Questions on Chemical Kinetics
For the two parallel reactions and , show that the activation energy ' for the disappearance of is given in terms of activation energies and for the two paths by
For the mechanism
Derive the rate law using the steady-state approximation to eliminate the concentration of .
Assuming that , express the pre-exponential factor and for the apparent second-order rate constant in terms of and and and for the three steps.
The reaction of formation of phosgene from and is . The proposed mechanism is
| (i) | (fast equilibrium) | |
| (ii) | (fast equilibrium) | |
| (iii) | (slow) |
Shows that the above leads to the following rate law
Where
The decomposition of a compound , at temperature according to the equation
is the first order reaction. After minutes from the start of decomposition in a closed vessel, the total pressure developed is found to be and after a long period of time the total pressure observed to be . Calculate the total pressure of the vessel after minute, if volume of liquid is supposed to be negligible. Also calculate the time fraction
Given : Vapour pressure of at temperature .
The catalytic decomposition of formic acid may take place in two ways:
(a)
(b)
The rate constant and activation energy for reaction (a) are at and respectively and for reaction (b) are at and respectively. Find the temperature which will give a product made up of equimolar quantities of water vapour, carbon monoxide, hydrogen and carbon dioxide.
For the following first order gaseous reaction
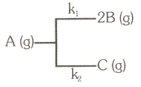
The initial pressure in a container of capacity litters is . Pressure at time is and after infinite time it becomes atmosphere. Find the rate constant and for the appropriate reactions.
