HARD
JEE Advanced
IMPORTANT
Earn 100
The standard state Gibb's free energies of formation of (graphite) and (diamond) at are
The standard state means that the pressure should be 1 bar, and substance should be pure at a given temperature. The conversion of graphite [ (graphite)] to diamond [ (diamond)] reduces its volume by . If (graphite) is converted to (diamond) isothermally at , the pressure at which (graphite) is in equilibrium with (diamond), is
[Useful information: ]
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Thermodynamics
HARD
JEE Advanced
IMPORTANT
For a reaction taking place in a container in equilibrium with its surroundings, the effect of temperature on its equilibrium constant in terms of change in entropy is described by:
HARD
JEE Advanced
IMPORTANT
When 100 mL of 1.0 M HCl was mixed with 100 mL of 1.0 M NaOH in an insulated beaker at constant pressure, a temperature increase of was measured for the beaker and its contents (Expt.1). Because the enthalpy of neutralization of a strong acid with a strong base is a constant , this experiment could be used to measure the calorimeter constant. In a second experiment (Expt. 2), 100 mL of 2.0 M acetic acid was mixed with 100 mL of 1.0 M NaOH (under identical conditions to Expt. 1) where a temperature rise of was measured. (Consider heat capacity of all solutions as and density of all solutions as )
Enthalpy of dissociation of acetic acid obtained from the Expt. 2 is
MEDIUM
JEE Advanced
IMPORTANT
For the process at and atmosphere pressure, the correct choice is-
HARD
JEE Advanced
IMPORTANT
An ideal gas undergoes a reversible isothermal expansion from state to state followed by a reversible adiabatic expansion from state to state The correct plot representing the changes from state to state is(are)
(: pressure, : volume, : temperature, : enthalpy, : entropy)
HARD
JEE Advanced
IMPORTANT
Choose the reaction(s) from the following options, for which the standard enthalpy of reaction is equal to the standard enthalpy of formation.
HARD
JEE Advanced
IMPORTANT
A reversible cyclic process for an ideal gas is shown below. Here, are pressure, volume and temperature, respectively. The thermodynamic parameters are heat, work, enthalpy and internal energy, respectively.

The correct option(s) is (are)
HARD
JEE Advanced
IMPORTANT
A closed tank has two compartments A and B, both filled with oxygen (assumed to be ideal gas). The partition separating the two compartments is fixed and is a perfect heat insulator (Figure). If the old partition is replaced by a new partition which can slide and conduct heat but does not allow the gas to leak across (Figure ), the volume (in ) of the compartment A after the system attains equilibrium is . Write the value of .
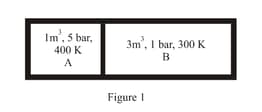
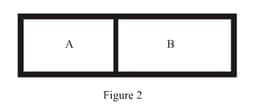
MEDIUM
JEE Advanced
IMPORTANT
An ideal gas is expanded from under different conditions. The correct statement(s) among the following is(are)
