The symbol of the aluminium atom is . Bohr model of the atom is given in the figure; analyse this and complete the table.
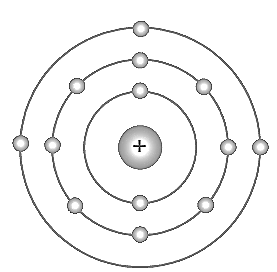
Atomic Number
Mass Number
Number of Protons
Number of Electrons
Number of Neutrons
Electronic configuration


Important Questions on Structure of Atom
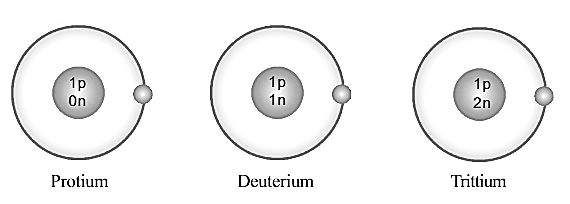
Complete the following table providing details related to these atoms.
| Name of Atom | Protium | Deuterium | Tritium |
| Number of Protons | |||
| Number of Neutrons | |||
| Number of Electrons | |||
| Atomic Number | |||
| Mass Number |
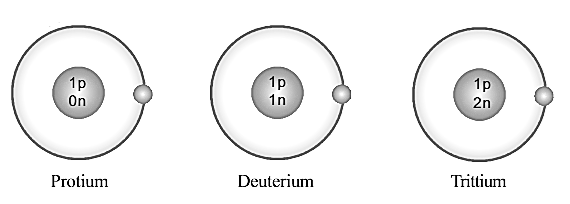
Which is the particle that differs in its number in these atoms?
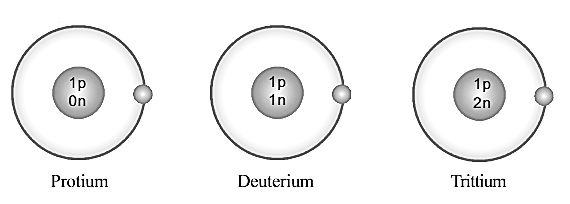
What interference can you arrive at when the mass number and an atomic number of these elements are examined?
Symbols of certain elements are given in the table. Complete the table writing there atomic number, mass number, number of protons, electrons and neutron.
| Symbol | Atomic Number | Mass Number | Protons | Electrons | Neutrons |
The atomic number of an atom Z= , mass number A= .
Find the number of protons, electrons and neutrons in the atom.
The atomic number of an atom Z=, mass number A=.
Write the electronic configuration of the atom.
The atomic number of an atom Z= , mass number A= .
Draw the Bohr model of an atom.
