The type of bonds present in are

Important Questions on Chemical Bonding and Molecular Structure
Choose the correct order of polarisability for the following:
In an ionic bond, the cation tends to polarise the electron cloud of the anion by pulling electron density towards itself. This causes development of covalent character in the ionic bond because the electron density gets localized in between the nuclei. The tendency of the cation to bring about the polarization of the anion is expressed as its polarising power. The ability of ion to undergo polarization is called its polarisability.
Which of the following statement is correct?
Arrange the following species in decreasing order of polarising powers: .
In an ionic bond, the cation tends to polarise the electron cloud of the anion by pulling electron density towards itself. This causes development of covalent character in the ionic bond because the electron density gets localized in between the nuclei. The tendency of the cation to bring about the polarization of the anion is expressed as its polarising power. The ability of ion to undergo polarization is called its polarisability.
The correct order of decreasing polarity is:
In an ionic bond, the cation tends to polarise the electron cloud of the anion by pulling electron density towards itself. This causes development of covalent character in the ionic bond because the electron density gets localized in between the nuclei. The tendency of the cation to bring about the polarization of the anion is expressed as its polarising power. The ability of ion to undergo polarization is called its polarisability.
Dative bond is present in:
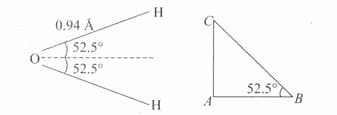
Dipole moment of is . If bond angle is and bond length is , determine magnitude of the charge on the oxygen atom in the water molecule.