HARD
JEE Main
IMPORTANT
Earn 100
The value of '' for steam is . The density of liquid water is at . What percentage of volume of water molecules occupy in gaseous phase of water in liquid phase?

Important Questions on States of Matter
HARD
JEE Main
IMPORTANT
Determine the pressure exerted by of in vessel at using van der Waals equation. Also report the pressure of gas, if it behaves ideally in nature. Given .
MEDIUM
JEE Main
IMPORTANT
Calculate the compressibility factor for carbon dioxide, if one mole of it occupies at and . Comment on the result.
HARD
JEE Main
IMPORTANT
For a given real gas, the compressibility factor is at and . Calculate the mass of gas required to fill a gas cylinder of capacity under given conditions. [Molar mass of gas ]
MEDIUM
JEE Main
IMPORTANT
At Boyle's temperature, the value of the compression factor has a value of one over a wide range of pressure. This is due to the fact that in the van der Waals equation.
EASY
JEE Main
IMPORTANT
Which of the following expressions is correct between the van der Waals constant and the radius of spherical molecules?
MEDIUM
JEE Main
IMPORTANT
The value of compressibility factor of a Van der Waal's gas at critical point is:
HARD
JEE Main
IMPORTANT
The pressure exerted by of at is , assuming that volume occupied by molecules is negligible. The value of Van der Waal's constant for attraction of gas is:
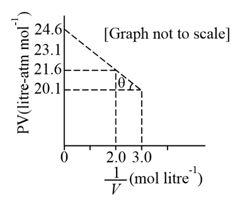
HARD
JEE Main
IMPORTANT
For one mole of a Van der Waal's gas when, and , the vs plot is shown below. The value of the Van der Waal's constant is: