EASY
NEET
IMPORTANT
Earn 100
The volume of one mole gas changes according to the relation . If temperature change is , then work done will be,
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
NEET
IMPORTANT
A cyclic process is shown in the diagram. Which of the following curves show the same process in a diagram?
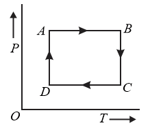
EASY
NEET
IMPORTANT
An ideal gas goes from state to state via three different processes as indicated in the diagram.
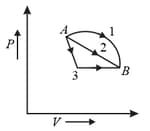
If , and indicate the heat absorbed by the gas along the three processes and , and indicate the change in internal energy along the three processes, respectively, then
MEDIUM
NEET
IMPORTANT
An ideal monoatomic gas undergoes a cyclic process as shown in the figure. The ratio of heat absorbed during to the work done on the gas during is
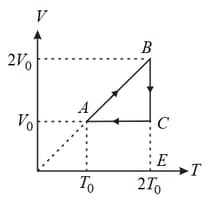
EASY
NEET
IMPORTANT
During an adiabatic process, the pressure of a gas is found to be proportional to the cube of its absolute temperature. The ratio for the gas is,
EASY
NEET
IMPORTANT
A gas undergoes an adiabatic process in which pressure becomes times and volume becomes times of its initial volume. If the initial absolute temperature was then, the final temperature is,
EASY
NEET
IMPORTANT
The work of is performed in order to compress one kilomole of a gas adiabatically and in this process the temperature of the gas increases by . The gas is
EASY
NEET
IMPORTANT
A diagram is shown below. Choose the corresponding diagram.
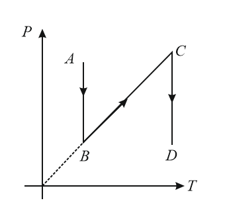
MEDIUM
NEET
IMPORTANT
The following are the diagrams for cyclic processes for a gas. In which of these processes heat is not absorbed by the gas?
