EASY
JEE Main/Advance
IMPORTANT
Earn 100
There is a mixture of nitrogen and helium kept at room temperature. As compared to a helium molecule, the nitrogen molecule hits the wall,
(a)with greater average speed.
(b)with smaller average speed.
(c)with greater average kinetic energy.
(d)with smaller average kinetic energy.
50% studentsanswered this correctly

Important Questions on Kinetic Theory of Gases
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
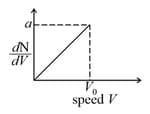
Graph shows a hypothetical speed distribution for a sample of gas particle .
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
