EASY
NEET
IMPORTANT
Earn 100
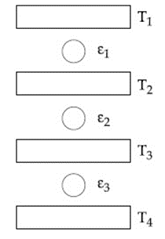
Three Carnot engines operate in series between a heat source at a temperature and a heat sink at temperature (see figure). There are two other reservoirs at temperature and as shown with The three engines are equally efficient if:
50% studentsanswered this correctly
Important Questions on Thermodynamics
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
