MEDIUM
NEET
IMPORTANT
Earn 100
Two substances and are present such that . The half-life of is minutes and of is minutes. If they start decaying at the same time, following first-order kinetics, after how much time will the concentration of both become equal?
(a) minutes
(b) minutes
(c) minutes
(d)minutes
54.55% studentsanswered this correctly

Important Questions on Chemical Kinetics
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
For a first-order reaction, the concentration of and at the point of intersection is given in the figure:
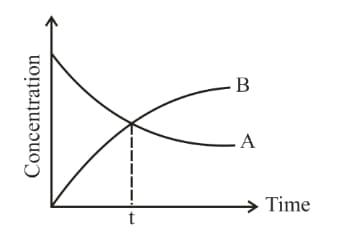
If mol at the time is equal to:
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
