HARD
11th CBSE
IMPORTANT
Earn 100
Use the following data to calculate for .
for sodium metal
Ionization enthalpy of sodium
Electron gain enthalpy of bromine
Bond dissociation enthalpy of bromine
for
for

Important Questions on Thermodynamics
MEDIUM
11th CBSE
IMPORTANT
MEDIUM
11th CBSE
IMPORTANT
EASY
11th CBSE
IMPORTANT
MEDIUM
11th CBSE
IMPORTANT
MEDIUM
11th CBSE
IMPORTANT
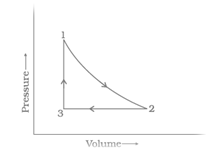
A sample of mol of a monoatomic ideal gas is taken through a cyclic process of expansion and compression as shown in the Figure. What will be the value of for the cycle as a whole?
EASY
11th CBSE
IMPORTANT
MEDIUM
11th CBSE
IMPORTANT
Identify the state functions and path functions out of the following :
enthalpy, entropy, heat, temperature, work, free energy.
EASY
11th CBSE
IMPORTANT
