EASY
AS and A Level
IMPORTANT
Earn 100
Use the information in Figure and the information below to demonstrate that the average bond energy of the bond is .
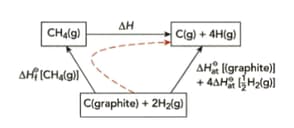


Important Questions on Enthalpy Changes
EASY
AS and A Level
IMPORTANT
Look at this equation.
.
Which one of the following statements is completely correct?
EASY
AS and A Level
IMPORTANT
Define standard enthalpy change of combustion.
MEDIUM
AS and A Level
IMPORTANT
When red phosphorus burns in oxygen the enthalpy change is . For white phosphorus the enthalpy change is . For both forms of phosphorus the reaction taking place is: .
Use this information to calculate the enthalpy change for the transformation: .
EASY
AS and A Level
IMPORTANT
Represent these changes on an energy level diagram.
EASY
AS and A Level
IMPORTANT
Define standard enthalpy change of formation.
EASY
AS and A Level
IMPORTANT
Calculate the standard enthalpy change of formation of methane from the following standard enthalpy changes of combustion:
EASY
AS and A Level
IMPORTANT
Calculate the standard enthalpy change of combustion of methane using the following bond energies:
EASY
AS and A Level
IMPORTANT
Define average bond energy.
