What happens when nitric acid is added to egg shell?

Important Questions on Acids, Bases and Salts
How would you distinguish between baking powder and washing soda by heating?
Salt Commonly used in bakery products on heating gets converted into another salt which itself is used for removal of hardness of water and a gas is evolved. The gas when passed through lime water, turns it milky. Identify and .
In one of the industrial processes for manufacture of sodium hydroxide a gas is formed as by-product. The gas reacts with lime water to give a compound which is as a bleaching agent in chemical industry. Identify and giving the chemical equation of the reaction involved.
Fill in the missing data in the given table.
| Name of the salt | Formula | Salt obtained from | ||
|
|
Base |
Acid | ||
| Ammonium chloride | -- | |||
| Copper sulphate | -- | -- | ||
| Sodium chloride | -- | |||
| Magnesium nitrate | -- | |||
| Potassium sulphate | -- | -- | ||
| Calcium nitrate | -- | |||
What are strong and weak acids? In the following list of acids, separate strong acids from weak acids. Hydrochloric acid, citric acid, acetic acid, nitric acid, formic acid, sulphuric acid.
In the following schematic diagram for the preparation of hydrogen gas as shown in the figure, what would happen if the following changes are made?
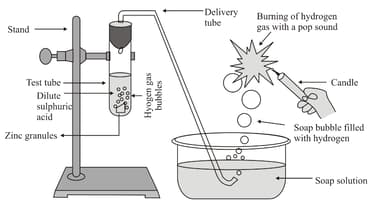
(a) In place of zinc granules, the same amount of zinc dust was taken in the test tube.
(b) Instead of dilute sulphuric acid, dilute hydrochloric acid is taken.
(c) In place of zinc, copper turnings are taken.
(d) Sodium hydroxide is taken in place of dilute sulphuric acid and the tube is heated.
