What will be the molar mass of the dibasic acid if of a dibasic acid is neutralized by of solution.?
Important Questions on Redox Reactions
Given below are two statements: one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A) : An aqueous solution of when for volumetric analysis, its concentration should be checked before the use.
Reason (R) : On aging, solution absorbs atmospheric .
In the light of the above statements, choose the correct answer from the options given below.
The titration curve of weak acid vs. strong base with phenolphthalein as indicator is shown below. The
Given:
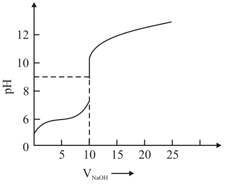
The number of following statement/s which is/are correct about phenolphthalein is _______
A. It can be used as an indicator for the titration of weak acid with weak base.
B. It begins to change colour at
C. It is a weak organic base
D. It is colourless in acidic medium
(equation not balanced). Few drops of concentrated were added to this solution and gently warmed. Further, oxalic acid ) was added in portions till the colour of the permanganate ion disappeared. What is the quantity of (in mg) present in the initial solution? (Atomic weights in )
of hypo solution is used for the titration of of copper sulphate solution, in the presence of excess of using starch as an indicator. The molarity of is found to be (nearest integer)
Given :
Statement I : In the titration between strong acid and weak base methyl orange is suitable as an indicator.
Statement II : For titration of acetic acid with $\mathrm{NaOH}$ phenolphthalein is not a suitable indicator.
In the light of the above statements, choose the most appropriate answer from the options given below:
Find the nonnality of solution, if of it is required to react completely with of solution. ( Molar mass of )

