HARD
JEE Main
IMPORTANT
Earn 100
When common salt is dissolved in water, the
(a)melting point of the solution increases.
(b)boiling point of the solution increases.
(c)vapour pressure of the solution decreases.
(d)None of these.
50% studentsanswered this correctly

Important Questions on Solutions
MEDIUM
JEE Main
IMPORTANT
Which of the following is (are) correct for non-volatile solute?
HARD
JEE Main
IMPORTANT
If and are the of a solvent and solution, respectively, and and are the mole of the solute and the solvent, then
MEDIUM
JEE Main
IMPORTANT
Vapour pressure – temperature curves of pure solvent and a solution containing a non-volatile solute are depicted in the figure aside. Select the correct statement(s) of the following.
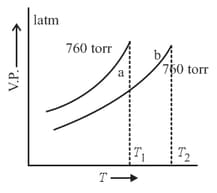
MEDIUM
JEE Main
IMPORTANT
The ebullioscopic constant depends upon
MEDIUM
JEE Main
IMPORTANT
How does sprinkling of salt help in clearing the snow-covered roads in hilly areas? Explain the phenomenon involved in the process.
HARD
JEE Main
IMPORTANT
If glycerin, , and methyl alcohol, , sell at the same price per pound, which one would be cheaper for preparing an antifreeze solution for the radiator of an automobile?
HARD
JEE Main
IMPORTANT
Camphor, , which has a freezing point of , has a freezing point depression constant of . Explain the usefulness and the limitations of camphor as a solvent for the determination of molecular weights. For what kind(s) of solute(s) would camphor be especially useful?
HARD
JEE Main
IMPORTANT
A certain solution of molal benzoic acid in benzene has a freezing point of and a normal boiling point of . The freezing point of benzene is and its boiling point is . Explain these observations, and suggest state for the solute particles at the two temperatures.
